Latest reviews
-
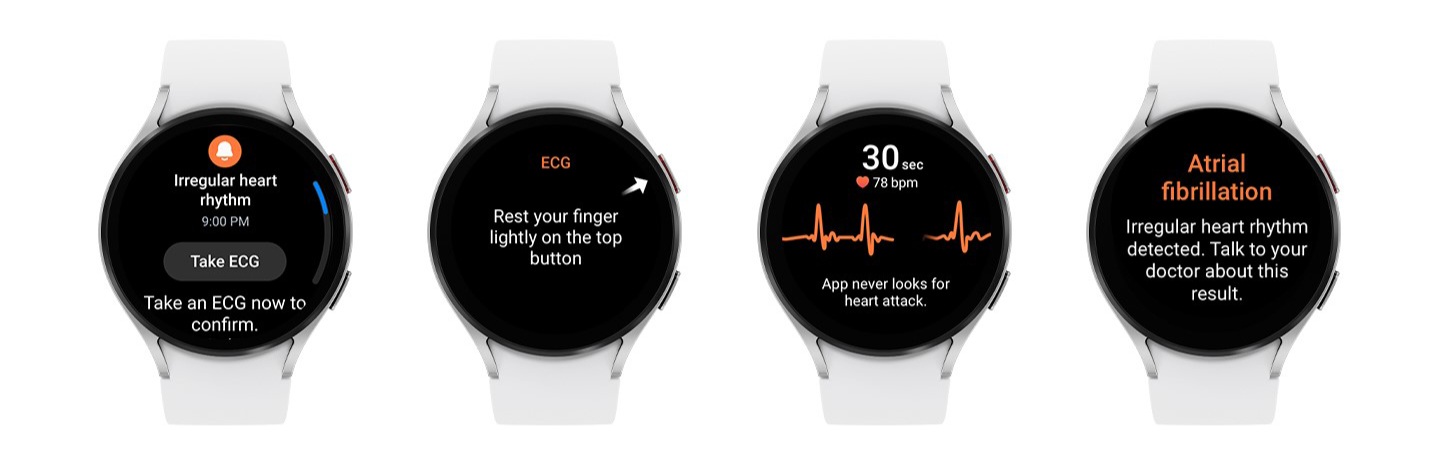
 Samsung Galaxy Watch 8 review
Samsung Galaxy Watch 8 review -
 Samsung Bespoke AI Jet Ultra review
Samsung Bespoke AI Jet Ultra review -
 Samsung Galaxy Z Fold 7 review
Samsung Galaxy Z Fold 7 review -
 Samsung Galaxy Z Flip 7 review
Samsung Galaxy Z Flip 7 review -
 Samsung Galaxy S25 Edge review
Samsung Galaxy S25 Edge review -
 Samsung S95F OLED TV review
Samsung S95F OLED TV review -
 Samsung Q7F QLED TV review: A no-brainer purchase at its low price
Samsung Q7F QLED TV review: A no-brainer purchase at its low price -
 Samsung S90F OLED TV review: Unreal value for money
Samsung S90F OLED TV review: Unreal value for money -
 Samsung Galaxy S25+ review: Nails the big phone basics
Samsung Galaxy S25+ review: Nails the big phone basics